A. |
Summary of this Application Note |
|
This Application Note describes how to use an immunoassay specific cleanup
method to prepare fly ash samples for screening analysis at 1 ppb using the CAPE Technologies High Performance Dioxin/Furan Immunoassay
Kit. The procedure is summarized below in section B
and is described in detail in section C.
Information on interpretation of results is given in section D.
Validation data supporting this method are given in section E.
The procedure described in this Application Note eliminates most of the sample cleanup
required for GC-MS analysis. This Application Note is intended to be used in conjunction
with the Dioxin/Furan Immunoassay Kit insert (IN-DF1).
|
|
|
B.
|
Summary of Sample
Preparation and Immunoassay Analysis |
| 1. |
Acid treat fly ash sample, then wash, dry, and extract by
conventional method (such as 16 hour Soxhlet extraction with toluene), but using either
immunoassay compatible internal standards or no internal standards. |
| 2. |
Treat an aliquot of toluene extract with 7% SO3 in
conc. H2SO4, then extract 3 times with hexane. |
| 3. |
Exchange hexane extract to methanol using the procedure
described in the kit insert IN-DF1. |
| 4. |
Perform the immunoassay procedure as described in the kit
insert IN-DF1. |
| 5. |
Interpret the immunoassay results as described in
section D of this Application Note.
|
|
|
C.
|
Detailed Sample Preparation
Procedure |
| 1. |
Acid treat the fly ash sample, then wash, dry, and extract
by conventional method (such as 16 hour Soxhlet extraction
with toluene). Clean extract using normal chromatographic
or other procedures used for GC-MS analysis.
Important Note:
It is essential that the sample not contain mass labeled
internal standards which include crossreactive congeners
(see the immunoassay kit insert IN-DF1,
section D on specificity). For information on the use of
immunoassay compatible internal standards, consult CAPE
Technologies Technical Note TN-001.
|
| 2. |
For screening analysis of fly ash
at 1 ppb, it is necessary to use 10 mg of sample equivalent
for each immunoassay tube. The following procedure allows
analysis using single or duplicate EIA tubes with minimal
leftover sample. Use an amount of extract equivalent to 30
mg of original sample. This will ultimately be reconstituted
in 30 µL, allowing either one or two 10 µL aliquots to be
removed for immunoassay analysis. It may also possible to
use a larger volume of extract to allow significant leftover
sample. However, it may be necessary to proportionately increase
the other volumes in the oxidation procedure (steps 3-6 below)
to maintain excess oxidizer. In either case, the amount
of extract delivered to each immunoassay tube should be equivalent
to 10 mg of the original sample. |
| 3. |
Reduce the volume of the original toluene extract by
evaporation if necessary. Place the volume of toluene extract chosen in step 2 into a 4 mL
glass vial with Teflon lined cap. |
| 4. |
Add 1 mL of hexane, then 1.0 to 1.5 mL of 7% SO3
in concentrated H2SO4. Cap vial and mix vigorously for at least 2
minutes. Centrifuge to separate phases completely (5 minutes or less at 1000 to 5000 x g). |
| 5. |
Remove as much hexane supernatant as possible without disturbing
the lower layer.
Caution: Do
not allow the lower phase to contaminate the hexane sample.
Any oxidizer which contaminates the sample at this point
will be carried through into the immunoassay, possibly leading
to invalid results.
Place the recovered hexane in a small round bottom glass
tube, such as 10 x 75 mm, or a small conical vial. Either
shape permits efficient recovery of the small reconstitution
volume in step 9.
|
| 6. |
Repeat the hexane addition, mixing, centrifugation, and
supernatant removal of steps 4 and 5 twice more for a total of 3 hexane extractions. It is
not necessary to add more 7% SO3 in concentrated H2SO4
after the first extraction. |
| 7. |
Add an aliquot of methanol + 100 ppm Triton X-100 (see
immunoassay kit insert IN-DF1,
section I.1) to the hexane sample. The aliquot volume should be the same as the planned
reconstitution volume (30 µL or larger volume if chosen). |
| 8. |
Evaporate the hexane samples at room temperature under a
gentle stream of nitrogen as described in the immunoassay kit insert IN-DF1, section I. |
| 9. |
Refer now to section I.6 of the immunoassay kit insert (IN-DF1). Redissolve the sample by
adding methanol to give the same volume as methanol-Triton in step 7 above. Perform this
step only after the EIA tubes have been prepared for standard and sample addition
according to the immunoassay kit insert IN-DF1,
section J, 1-4. |
| 10. |
Perform the immunoassay procedure as described in the DF1 Kit
insert IN-DF1,
section J, 5-11.
|
|
|
D.
|
Interpretation of
Immunoassay Results |
| 1. |
Calculate each optical density (OD) reading as a percent of
the negative control OD reading (%NC). Refer to the immunoassay kit insert IN-DF1, section D (Table 1), for
comparison of standard results to acceptable ranges. If your standards are not within
these ranges, your results may be invalid. |
| 2. |
Samples which have %NC values greater than standard 2 (10
pg/tube) contain less than 1 ppb TEQ in the original sample. Remember that there is an
inverse relationship between OD and concentration. Less color means a higher concentration
of PCDD/Fs. |
| 3. |
Samples which have %NC values less than standard 2 (10
pg/tube) contain more than 1 ppb TEQ in the original sample. |
| 4. |
Samples which have %NC values the same as standard 2 (10
pg/tube) contain approximately 1 ppb TEQ in the original sample. Consult the immunoassay
kit insert IN-DF1,
Table 3, for guidance in deriving additional screening information from your results. |
| 5. |
For suggestions on incorporation of these results into an
immunoassay based screening program, consult CAPE Technologies
Technical Note TN-002.
|
| 6. |
Advanced analysts may wish to use the CAPE Technologies High
Performance Dioxin/Furan Immunoassay Kit to produce quantitative results. To understand
the requirements and limitations of this approach, read CAPE
Technologies Technical Note TN-004.
|
|
|
E.
|
Validation Data Supporting this Method |
|
Incinerator fly ash samples were analyzed by conventional HRGC-HRMS.
Subsamples of the same 25 ash samples were extracted separately, without mass-labeled
internal standards. These samples were analyzed by immunoassay using the protocol
described in section C. Two different
comparisons of the results from the two methods are given in the following figures. These
results clearly establish the ability of the CAPE Technologies
High Performance Dioxin/Furan Immunoassay Kit to analyze TEQ in oxidized crude fly ash
extracts. |
Figure 1. Correlation between quantitative immunoassay analysis
and TEQ as determined by high resolution gas chromatography-high resolution mass
spectrometry (HRGC-HRMS) for 25 oxidized crude fly ash extracts. The calculated regression
line is shown with 99% confidence limits. These results clearly establish the ability of
the immunoassay to measure TEQ in oxidized crude fly ash extracts in the range from high
ppt to high ppb.
FIGURE
1 TO BE ADDED LATER
|
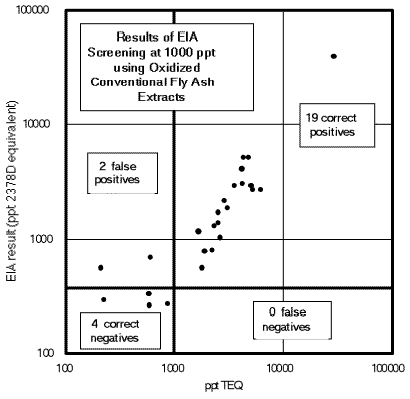
Figure 2. Quantitative EIA results for 25 oxidized crude fly
ash extracts plotted in screening format. The same data as used in Figure 1 were plotted
and overlaid with lines indicating 1) the TEQ value chosen as a screening level (1 ppb
vertical line) and 2) the EIA response at which the screening decision would be made (0.5
ppb horizontal line). The number of results in each quadrant is indicated on the plot.
These results clearly establish the ability of the CAPE Technologies High Performance
Dioxin/Furan Immunoassay Kit to screen oxidized crude fly ash extracts at 1 ppb TEQ.
 |
|
|
|